Americans struggling to find a therapy that will control their seizures now have access to a new treatment. Epidiolex is a plant-derived pharmaceutical formulation of cannabidiol (CBD) oil that has received significant attention in recent years for its potential to treat intractable seizures. Its FDA approval is great news for patients with Lennox-Gastaut and Dravet syndromes—two hard-to-treat forms of epilepsy—and for many others with health conditions causing seizures.
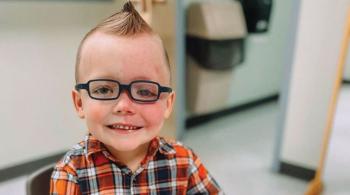
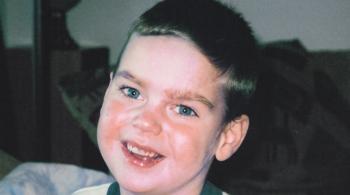
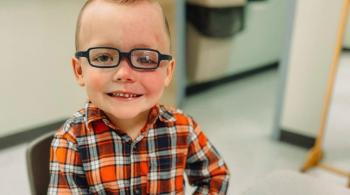
In a recent study to evaluate Epidiolex in the treatment of pediatric epilepsy, Dr. Comi and her team at the Kennedy Krieger Institute found the drug might also reduce seizure frequency in children with Sturge-Weber syndrome (SWS), a vascular, non-familial disorder caused by gene mutation and typically characterized by a congenital facial birthmark and neurological abnormalities.
The study, titled “Cannabidiol Treatment for Refractory Seizures in Sturge-Weber Syndrome,” identified children with SWS and treatment-resistant epilepsy. Each study participant received CBD twice a day. The study, part of an expanded-access program approved by the FDA, concluded with three patients receiving the administered drug for greater than one year.
During the course of the study, all three patients demonstrated a greater-than-50-percent reduction in seizures, and one of the three became seizure-free. Going into the study, all three had significant cognitive, neurological, behavioral and/or mood disorders, and each one was prescribed two or more anticonvulsants and low-dose aspirin at the beginning of the study. These findings represent the first of many studies needed to fully understand how CBD treatment can affect patients with SWS.
Today’s approval of Epidiolex brings the medical profession one step closer to being able to provide effective treatment options to patients suffering from Lennox-Gastaut and Dravet syndromes. And while more research is needed, recent studies like Kennedy Krieger’s Epidiolex study suggest CBD may have broad potential to effectively treat patients struggling with a variety of other conditions causing seizures, like SWS.
* Material support in the form of the study drug was provided by GW Research. GW Research also provided administrative assistance, as well as measurement and analysis of levels of CBD and its metabolites. GW Research’s medical, legal and regulatory team completed a review of the manuscript for intellectual property protection and accuracy of product description. All comments around clarity of writing and style are suggestions only, and are offered as a professional courtesy. A
About Kennedy Krieger Institute
Internationally recognized for improving the lives of children and adolescents with disorders and injuries of the brain, spinal cord and musculoskeletal system, Kennedy Krieger Institute in Baltimore, Maryland, serves 24,000 individuals a year through inpatient and outpatient clinics, home and community services, and school-based programs. Kennedy Krieger provides a wide range of services for children with developmental issues, from mild to severe, and is home to a team of investigators who are contributing to the understanding of how disorders develop, while at the same time pioneering new interventions and methods of early diagnosis. Visit KennedyKrieger.org for more information about Kennedy Krieger.
# # #