Disrupted social behavior is a core feature of compromised mental health, including anxiety and depression, and is a long-standing early diagnostic marker of disorders that emerge in later-life. Yet, we have little understanding of the ontogeny of social behavior neural circuits or how environmental perturbation at different stages of development impacts infant behavior.
Using the infant rodent pup, our lab studies the specific infant neuroanatomical circuits that generate developmentally-appropriate social behavior and how these systems go awry following adversity. Our team’s work examines how ethologically-relevant social challenges, such as social hierarchy disruption and abusive caregiving during infancy, produce profound changes in brain structure and function to modify behavior.
Lab Focus 1: Neural circuits supporting flexible social behavior in typical and perturbed development
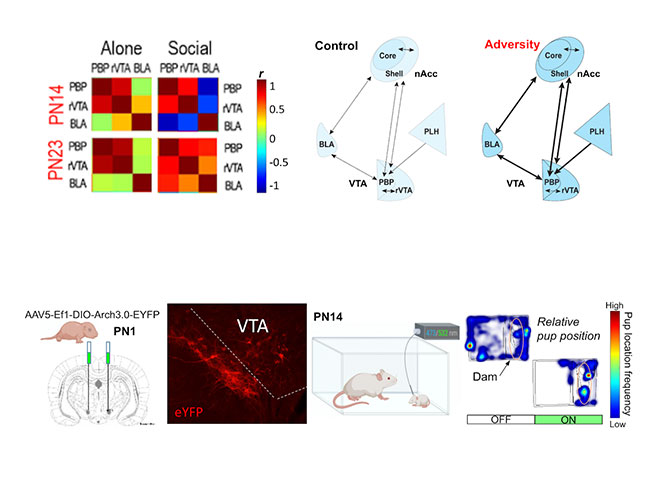
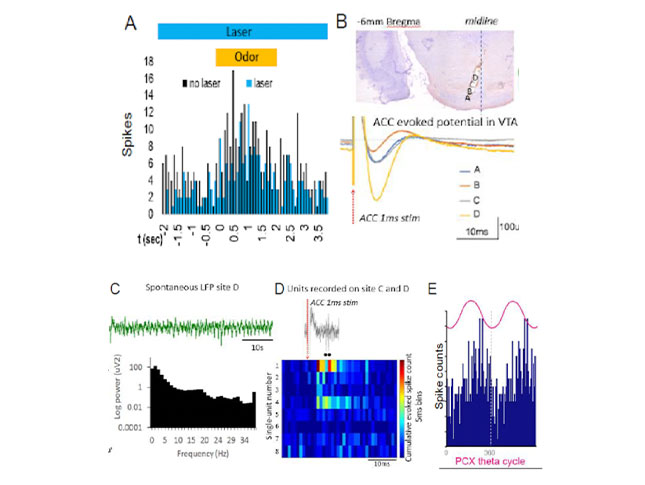
Due to technical limitations, it has been previously challenging to functionally dissect social behavior circuits in the behaving infant. My research program will uses state-of-the-art neuroscience techniques I have adapted for pups to dissect functional transitions in developing amygdala circuits known to regulate social behavior in adults. Using optogenetics in awake-behaving infant rat pups, we recently showed that dopaminergic innervation of pup BLA bidirectionally controls social approach behavior towards the mother and peers (Opendak et al., Neuron 2021). Our lab assesses this developing circuitry using multi-unit electrophysiology, fiber photometry, and optogenetic-guided manipulation of neural ensembles and behavior in pups to directly interrogate the role of the amygdala and its modulation by dopamine from the ventral tegmental area (VTA) in freely behaving rat pups at two ages: PN14 pups dependent upon the mother and PN23 weaning-age pups. We focus on cross-structural neural oscillatory coupling, which has gained attention as a potential mechanism by which the brain binds activity of regionally distributed populations of neurons to generate behavior.
Lab Focus 2: Sensory processing of social cues promoting flexible social behavior across development
Our second major research focus has been to understand how maternal care during adversity-rearing impacts the infant brain — an impossible experiment to conduct in humans and a novel approach in animal models. This work demonstrated that chronic stress in the caregiver’s presence produced amygdala structural abnormalities, heightened amygdala engagement during social behavior, and perturbed social approach toward the caregiver (Raineki, Opendak et al., PNAS 2019). In the nest, we observed blunted cortical processing of nurturing maternal behaviors (e.g., nursing, grooming), while cortical responses to rough handling were unaffected (Opendak et al., Nature Communications 2020).
A clear outcome that emerges from this research is the idea that social adversity perturbs neural processing of socially-relevant cues. We now aim to characterize typical and perturbed sensory processing of socially salient inputs on the level of units, ensembles and circuits across early development. Using optogenetic circuit dissection and cross-structural electrophysiological recordings, I will assess age- and experience-dependent plasticity in circuits engaged in social cue processing and valence (e.g. piriform-BLA, BLA-mPFC, VTA-lateral habenula). Implementing these cutting-edge measures in the pup will help characterize neurophysiological plasticity supporting social processing across development and identify loci of dysfunction following adversity.
Lab Focus 3: Circuit-based analysis of ongoing repair mechanisms in the infant brain
While a rich literature has documented links between early adversity and later-life outcomes, environmental interventions in early life represent a promising therapeutic approach for recovery after trauma. We have collected data suggesting that providing the mother with opportunities for social interaction and a running wheel after adversity prevents pups’ elevated BLA-CeA functional connectivity, amygdala LFP gamma power and social deficits (Fig. 4). We are now leveraging our recent technical advances in infant circuit dissection to characterize mechanisms of repair during the intervention. To assess ongoing repair mechanisms, we combine optogenetic control of amygdala circuits with continuous LFP recordings in the nest to characterize pup brain dynamics before, after, and during intervention. This permits us to track the effects of repair and target circuits within the same animal.

