Biographical Sketch

Dr. Hua received his master's (2005) and doctoral (2009) degrees in biomedical engineering and electrical engineering at the Johns Hopkins University. His doctoral training centered on the development of novel MRI technologies for in vivo physiological imaging in the brain, such as protein content and cerebral blood volume.
After completing a post-doctoral fellowship in the Department of Radiology at the Johns Hopkins University from 2009 to 2010, Dr. Hua became a faculty member in the Department of Radiology at Johns Hopkins University School of Medicine and Kennedy Krieger Institute.
Research Summary
Dr. Hua’s research has centered on the development of novel MRI technologies for in vivo functional and physiological imaging in the brain, and the application of such methods for studies in healthy and diseased brains. These include the development of human and animal MRI methods to measure functional brain activities, cerebral perfusion and oxygen metabolism at high (3 Tesla) and ultra-high (7 Tesla and above) magnetic fields. He is particularly interested in novel MRI approaches to image small blood and lymphatic vessels in the brain. Collaborating with clinical investigators, these techniques have been applied 1) to detect functional, vascular and metabolic abnormalities in the brain in neurodegenerative diseases such as Huntingdon’s disease (HD), Parkinson’s disease (PD), Alzheimer’s disease (AD) and mental disorders such as schizophrenia; and 2) to map brain functions and cerebrovascular reactivity for presurgical planning in patients with vascular malformations, brain tumors and epilepsy.
Imaging Technology Development
1. Inflow-based vascular space occupancy (iVASO) MRI
In the brain, the supply of adequate oxygen and energy substrates for local metabolic demands is controlled by blood vessels. Therefore, microvascular abnormalities often accompany metabolic disturbance in the brain, and have been associated with many neurodegenerative diseases. To date, most studies of the microvasculature in the brain using MRI measure total cerebral blood volume (CBV) and flow (CBF) in the brain, which reflects the sum of signals from the arterial and venous vessels in the microvasculature. However, different types of blood vessels have distinct functions and physiology, and can be affected differentially by pathology. The arterioles are the most actively regulated blood vessels, and thus may be more sensitive to metabolic disturbances in the brain. Therefore, the measurement of changes in each type of blood vessels in different segments of the microvasculature separately may furnish information that is not obtainable from total CBV and CBF measures, and may provide a more sensitive biomarker for brain diseases. The inflow-based vascular space occupancy (iVASO) MRI approach was developed in our group, which can measure CBV in small pial arteries and arterioles (CBVa) with diameters up to 100-150 microns. Unlike many existing MRI methods for CBV measurements, this is a truly non-invasive method, which does not require the administration of exogenous contrast agents. The iVASO method has been applied in several brain diseases such as stroke, brain tumors, Huntington’s disease, Alzheimer’s disease, Parkinson’s disease, and schizophrenia.
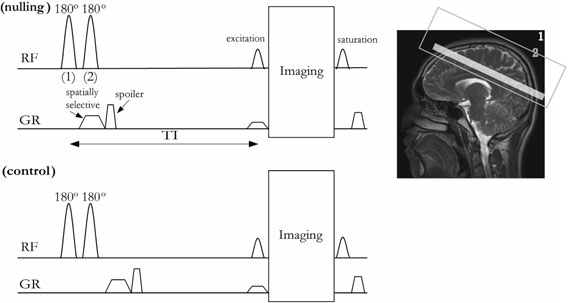
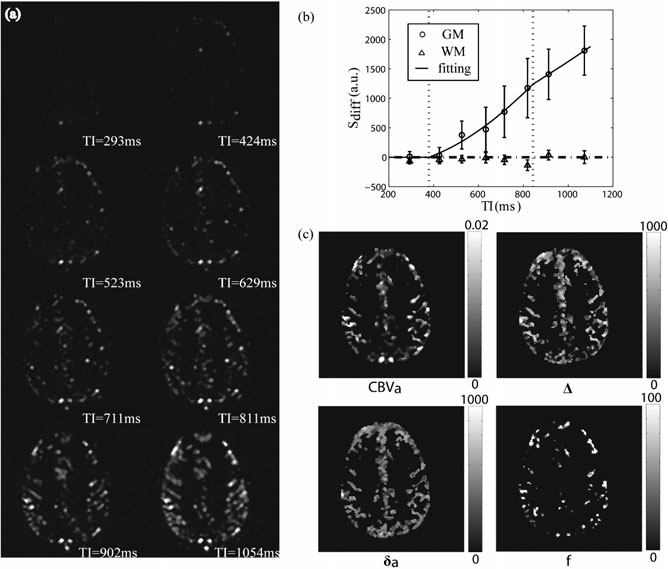
Figures from Hua et al., NMR in Biomedicine 24(10):1313-25, 2011.
2. T2-prepared (T2prep) BOLD fMRI and Diffusion-prepared DTI
Blood-oxygenation-level-dependent (BOLD) functional MRI (fMRI) has revolutionized the noninvasive assessment of brain function. Diffusion tensor imaging (DTI) is the only noninvasive imaging method that can visualize the trajectories of main white matter (WM) fascicles in vivo. Echo-planar-imaging (EPI) is currently the method of choice for most fMRI and DTI studies. However, the well-known geometric distortion and signal dropouts in EPI images caused by large magnetic susceptibility effects have hampered its application in some areas. In a normal brain, regions close to the skull base and paranasal sinuses at bone/soft tissue and bone/air interfaces are usually most affected by these susceptibility artifacts, which typically include the orbitofrontal regions and temporal lobes.
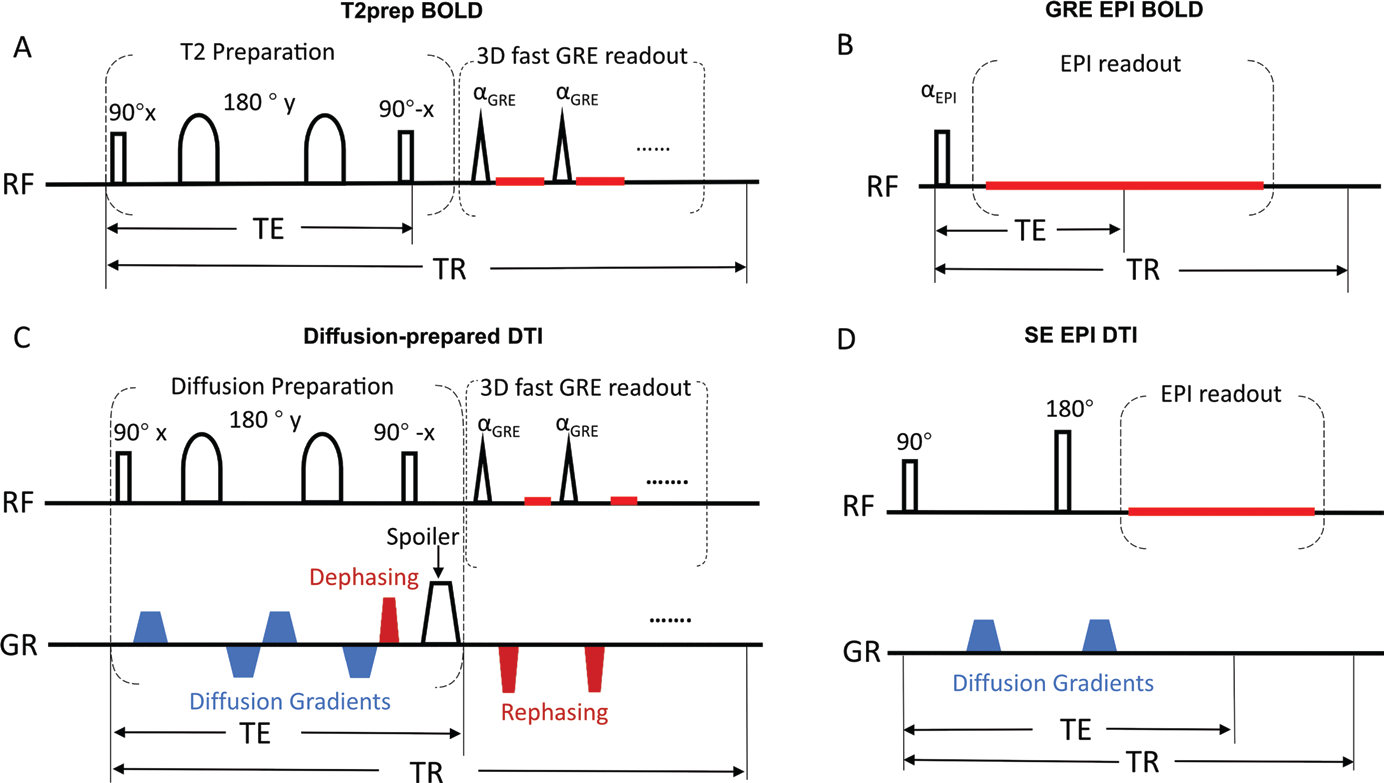
Figure: Pulse sequence diagram.
A whole-brain T2-prepared (T2prep) BOLD fMRI approach was developed in our group, which uses a spin preparation module (T2prep) before readout to induce T2-weighted BOLD effects for fMRI. Similarly, the diffusion sensitized driven equilibrium (DSDE) sequence was used for diffusion-prepared DTI. By adopting a 3D fast GRE readout with short TE, a sequence typically used in anatomical imaging, both sequences showed minimal distortion and dropout across the entire brain, which provided clear access to regions near air-filled cavities that are often inaccessible with conventional EPI readout due to susceptibility artifacts.
Figure: Typical images acquired using T2prep BOLD and GRE EPI (a) in a healthy brain, and (b) in a subject wearing dental braces.
Metallic Implants:
Furthermore, such susceptibility artifacts become more severe in the presence of MR compatible metal head implants, such as metallic dental fillings and braces and various other devices (MR compatible aneurysm clips, endovascular coils, etc.). We performed T2prep BOLD fMRI and diffusion-prepared DTI in healthy subjects wearing metallic dental braces to evaluate their ability to minimize susceptibility artifacts in the presence of metallic objects. Dental fillings and braces is particularly a problem for MRI studies involving teenagers as >80% teenagers in the US and 33% of the world’s population including adults have orthodontic treatments (www.aaoinfo.org). We demonstrated that T2prep BOLD fMRI and diffusion-prepared DTI can acquire functional and diffusion MR images, respectively, in healthy human subjects wearing metallic dental braces without the susceptibility artifacts commonly seen in conventional EPI images.
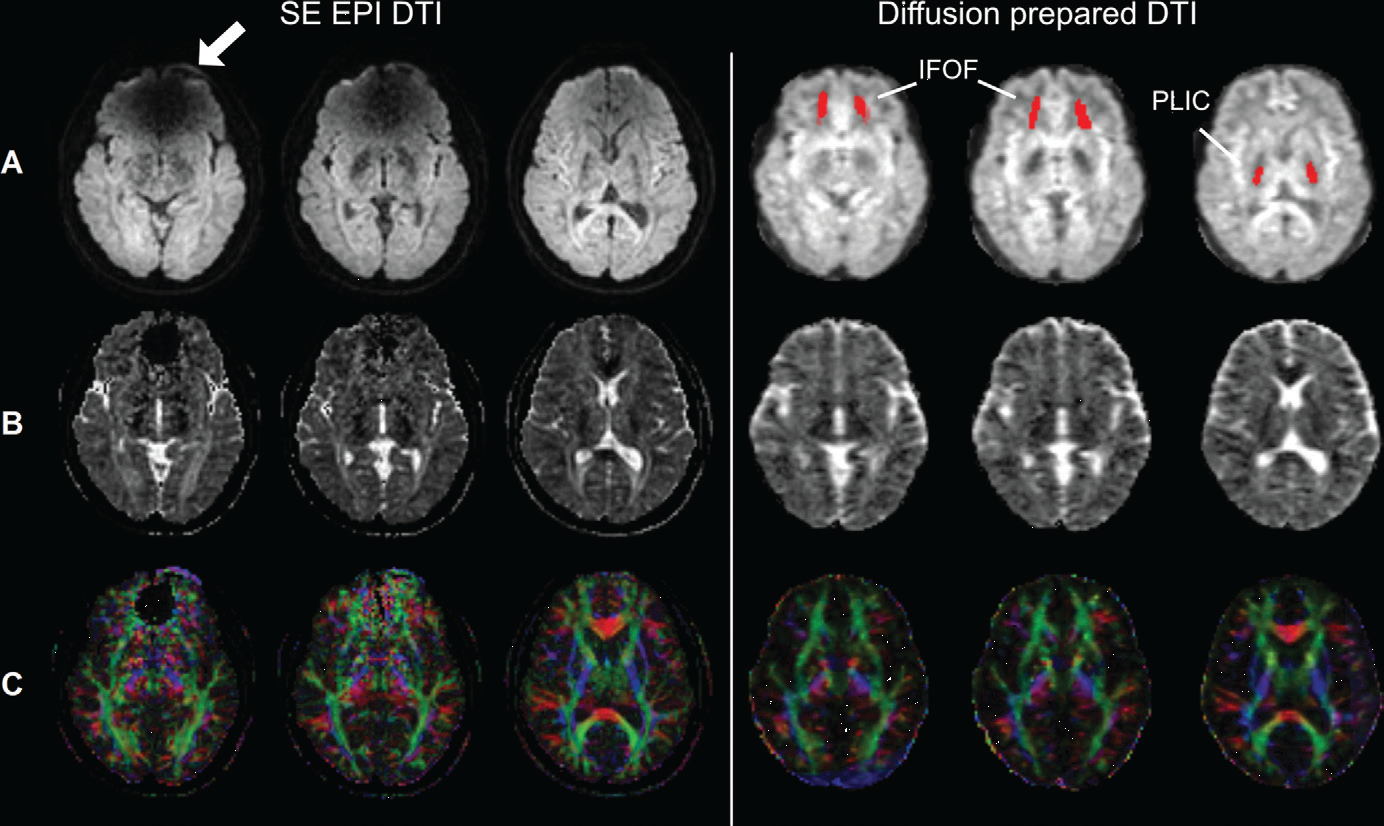
Figure from Miao et al., Radiology. 2020 Jan;294(1):149-157
Presurgical Brain Mapping:
Presurgical brain mapping using fMRI and DTI have been increasingly performed in large medical centers across the world. Presurgical functional mapping is currently the most prevalent clinical application of fMRI and is the only application of fMRI for which there are approved AMA (American Medical Association) billing CPT (Current Procedural Terminology) codes (http://www.asfnr.org/cpt-codes/). Presurgical fMRI is commonly used to noninvasively locate essential cortical sensorimotor and language areas and to determine the language dominant hemisphere prior to surgery, thus helping to reduce the need for invasive diagnostic procedures such as direct cortical stimulation and to provide complementary information. DTI furnishes essential anatomical information on specific white matter fascicles, and in the current era is an integral part of presurgical MRI. The ultimate goal of surgery is to achieve maximal removal of the pathology while preserving vital brain functions. To this end, preoperative fMRI and DTI can provide indispensable information for neurosurgeons for the planning of optimal treatment strategy on an individual basis.
A significant subpopulation of patients who need to undergo presurgical brain mapping are affected by the geometric distortion and signal dropout in regions with large magnetic susceptibility effects when using the current standard EPI based sequences. These include several regions near the skull base and air cavities such as the orbitofrontal and temporal cortex that are important for many cognitive and language functions, and brain areas close to MR- compatible metallic head implants. Furthermore, such susceptibility artifacts can also arise from cavities related to previous surgery, calcified structures, hemorrhage, and metal implants.
Using T2prep BOLD fMRI for presurgical mapping in epilepsy and brain tumor patients, it showed greater functional sensitivity around the lesions containing blood products and air-filled cavities compared to GRE EPI BOLD fMRI. Functional activation was detected with T2prep BOLD but not GRE EPI BOLD in the affected areas with the same functional tasks and statistical threshold.
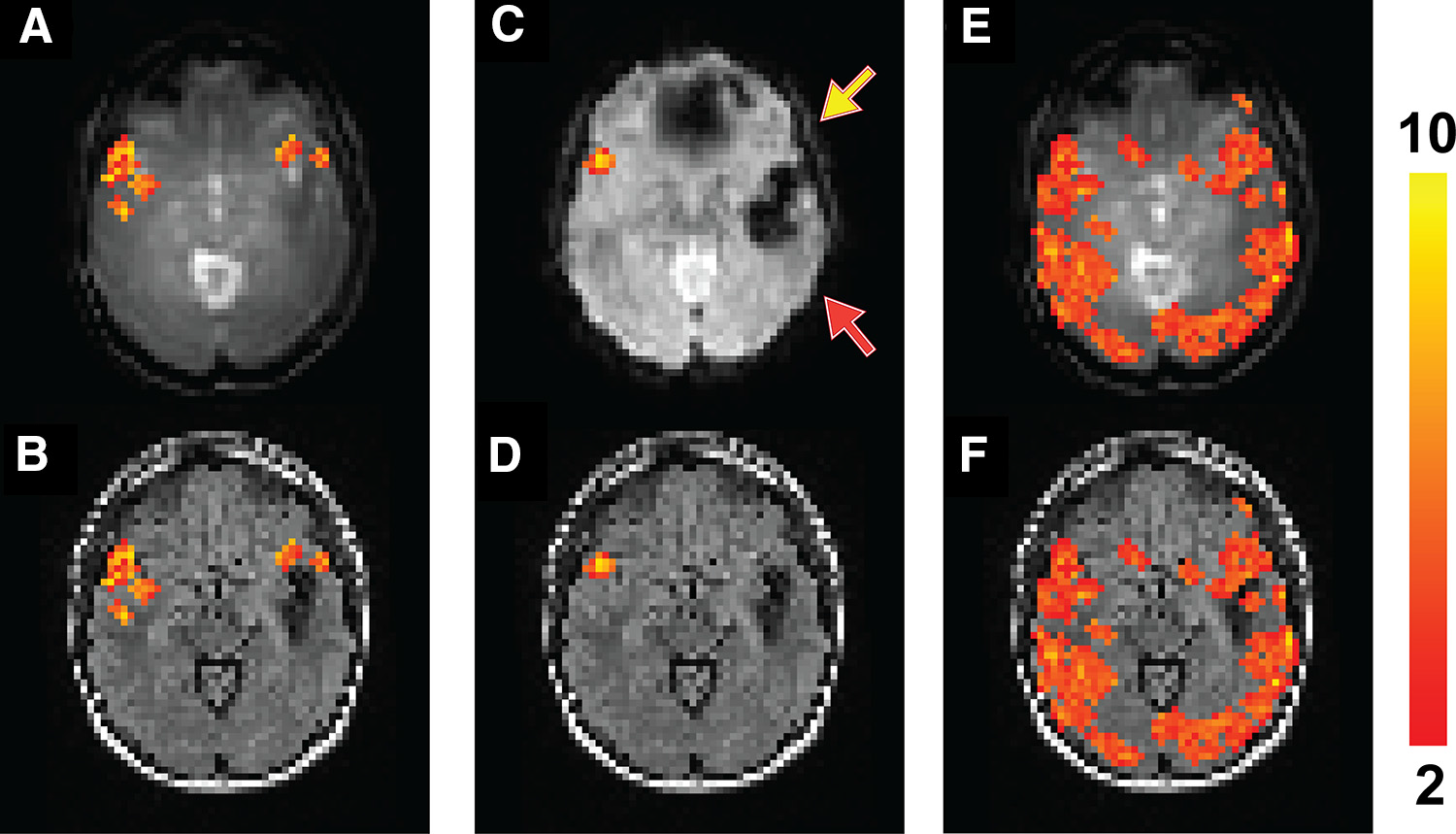
Figure from Hua et al., Tomography 2017 Jun;3(2):105-113. Presurgical brain mapping using fMRI in a patient with epilepsy with a large cavernous malformation in the left temporal lobe and a small one in the anterior left frontal lobe, causing signal dropouts in the areas in the EPI images (C). A sentence completion task was performed for language mapping. Activation in the left inferior frontal lobe (an important language region, yellow arrow) was detected in the T2prep BOLD scan (A) but not the GRE EPI BOLD scan (C).
Olfactory fMRI:
The olfactory bulb and several other regions associated with olfaction such as the piriform cortex (primary olfactory cortex) and orbitofrontal cortex are substantially affected by the susceptibility artifacts caused by the nearby nasal cavity. Using T2prep BOLD fMRI, functional activities can be detected in these regions including the olfactory bulb, which is inaccessible with conventional EPI sequences.
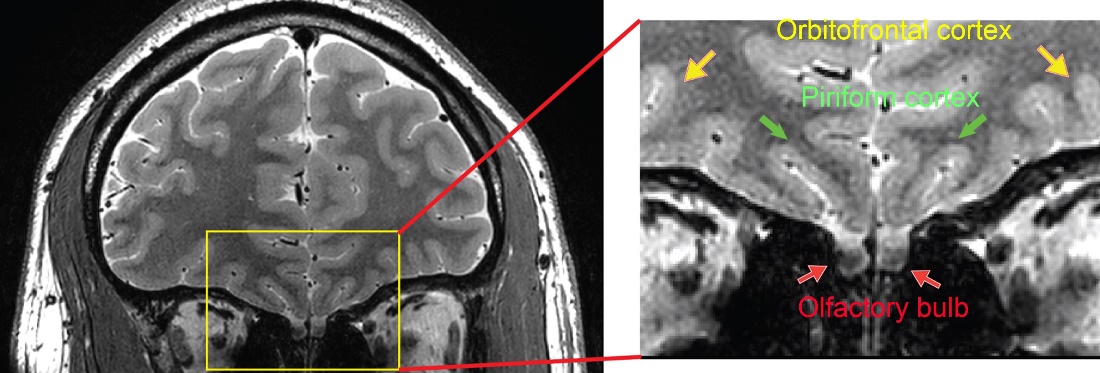
Figure: High resolution (0.5mm) structural images showing regions related to the olfactory system in the human brain.

Figure: A custom-built multi-channel computer-controlled olfactometer used to deliver the odorants in precisely timed pulses during fMRI scans.
Selected Publications:
- Hua J, Qin Q, Van Zijl PCM, Pekar JJ, Jones CK. Whole-brain three-dimensional T2-weighted BOLD functional magnetic resonance imaging at 7 Tesla. Magnetic Resonance in Medicine 2014 Dec;72(6):1530-40. PMC4055555.
- Hua J, Miao X, Agarwal S, Bettegowda C, Quiñones-Hinojosa A, Laterra J, Van Zijl PCM, Pekar JJ, Pillai JJ. Language mapping using T2-prepared BOLD functional MRI in the presence of large susceptibility artifacts – initial results in brain tumor and epilepsy patients. Tomography 2017 Jun;3(2):105-113. DOI: 10.18383/j.tom.2017.00006. PMC5552052.
- Miao X, Wu Y, Liu D, Jiang H, Woods D, Stern MT, Blair NIS, Airan RD, Bettegowda C, Rosch KS, Qin Q, van Zijl PCM, Pillai JJ, Hua J*. Whole-Brain Functional and Diffusion Tensor MRI in Human Participants with Metallic Orthodontic Braces. Radiology. 2020 Jan;294(1):149-157. doi: 10.1148/radiol.2019190070. Epub 2019 Nov 12. PubMed PMID: 31714192; PubMed Central PMCID: PMC6939835.
3. 3D-TRIP MRI
The gross signal change in BOLD fMRI does not have a clear physiological meaning. In fact, it may reflect an ensemble of changes in several physiological parameters, including cerebral blood volume (CBV) and flow (CBF), and cerebral metabolic rate of oxygen (CMRO2). A number of approaches have been developed to measure CBV and/or CBF responses along with the BOLD signal change, making it possible to calculate CMRO2 dynamics using quantitative BOLD theories. Conventionally, BOLD, CBV, and CBF signal changes are measured using consecutive MRI scans. In such approaches, the scan time is relatively long, and more importantly, there may be changes in physiological parameters between scans, causing inaccurate calculations of CMRO2. The 3D-TRiple-acquisitionafter-Inversion-Preparation (3D-TRIP) MRI approach was developed by our group, which can measure BOLD, CBF, and CBV signal changes in a single MRI scan with whole brain coverage. Using this approach, we were able to examine neurovascular and metabolic abnormalities in the brain in several brain diseases, such as Huntington’s disease (HD) and schizophrenia.
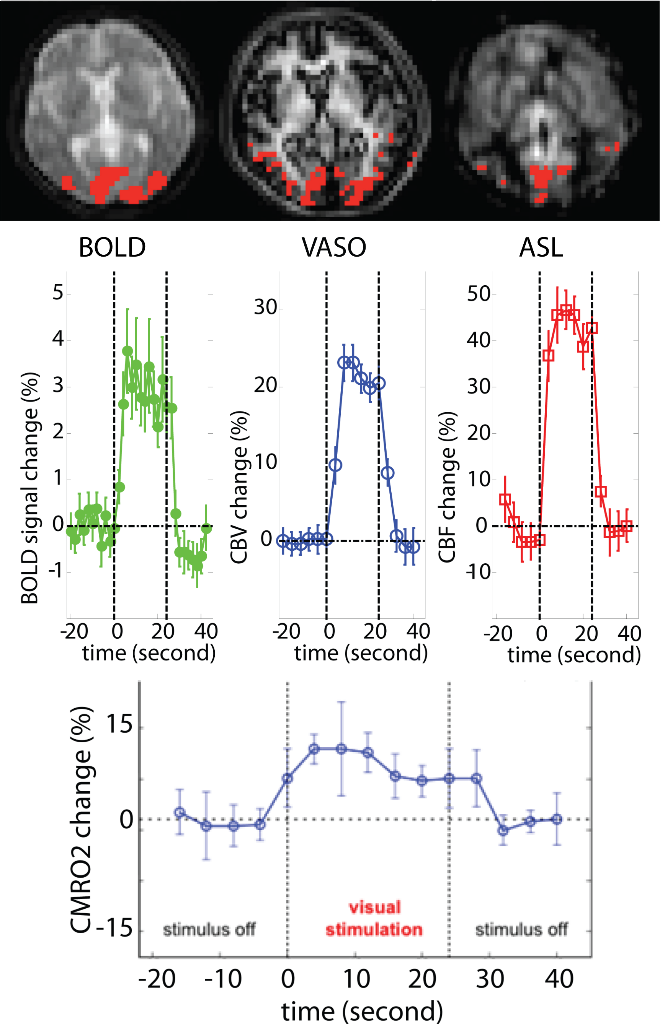
Figure: 3D-TRIP MRI in a healthy human subject.
Selected Publications:
- Cheng Y, Van Zijl PCM, Pekar JJ, Hua J*. Three-dimensional Acquisition of Cerebral Blood Volume and Flow Responses during Functional Stimulation in a Single Scan. Neuroimage 2014 Dec;103:533-41. PMC4252776.
- Cheng Y, Qin Q, Van Zijl PCM, Pekar JJ, Hua J*. A three-dimensional single-scan approach for the measurement of changes in cerebral blood volume, blood flow, and blood oxygenation-weighted signals during functional stimulation. Neuroimage, 147:976-984, 2017 Feb. PMID28041979.
- Klinkmueller P, Kronenbuerger M, Miao X, Bang J, Ultz KE, Paez A, Zhang X, Duan W, Margolis RL, Zijl PCV, Ross CA, Hua J*. Impaired response of cerebral oxygen metabolism to visual stimulation in Huntington's disease. J Cereb Blood Flow Metab. 2020 Aug 17;:271678X20949286. doi: 10.1177/0271678X20949286. [Epub ahead of print] PubMed PMID: 32807001.
4. Dynamic Imaging of Small Lymphatic Vessels in the Brain
The circulation of cerebrospinal fluid (CSF) influences various aspects of brain physiology, including substance distribution and waste clearance from the brain parenchyma. Recently, cerebral vessels with typical endothelial markers as lymphatic vessels in other organs in the body have been identified in the dura mater alongside the dural venous sinuses, in regions around the middle meningeal artery and cribriform plate, and in the basal part of the skull in animal models. Some of the meningeal lymphatic vessels have also been visualized in human brains. Cerebral lymphatic vessels are believed to play an important role in the drainage of CSF into the cervical lymph nodes, which has intriguing clinical implications for the clearancabnormal protein and other products in many brain diseases, such as Alzheimer's disease and Parkinson's disease.
The CSF space can be visualized on structural MR images with proper contrast adjusted to the much longer longitudinal (T1) and transverse (T2) relaxation times of CSF compared to the other tissues in the brain. When gadolinium(Gd)-based contrast medium are used, MR signal contrast between pre-contrast and post-contrast MR images can often be observed in the CSF at certain locations within the intra-cranial space. This is mainly due to the fact that the dural blood vessels lack a blood-brain barrier (BBB) that presents in cortical blood vessels, which enables some of the commonly used Gd contrast agents in human clinical MRI scans to cross the dural blood vessel wall and to enter the CSF. Recent studies have shown that some meningeal lymphatic vessels in the brain can be visualized using MRI with Gd contrast in human brains.
However, most existing approaches for the detection of Gd-based MR signal changes in the CSF and cerebral lymphatic vessels typically take at least a few minutes to achieve whole brain coverage and sufficient spatial resolution, which provides a relatively low temporal resolution. Moreover, few studies have performed a systemic investigation to optimize the contrast for the detection of Gd-based signal changes in the CSF (most Gd studies focus on Gd contrast in the blood). If the dynamic signal changes in the CSF and cerebral lymphatic vessels before and after Gd administration can be tracked with sufficient spatial and temporal resolution, it may serve as a useful tool for the investigation of CSF drainage routes in the brain, which has not been fully understood yet.
We recently developed an MRI sequence to image Gd-based signal changes in the CSF with a high temporal resolution (TR < 10 s), a fine spatial resolution (1-0.65 mm isotropic voxel) and whole brain coverage on 3T and 7T human MRI scanners. With this method, dynamic signal changes and Gd concentration can be quantified in the CSF space around several regions containing cerebral lymphatics vessels in the brain, such as the dural sinuses (superior sagittal sinus), middle meningeal artery, cribriform plate (olfactory region) and basal brain regions (jugular foramen).
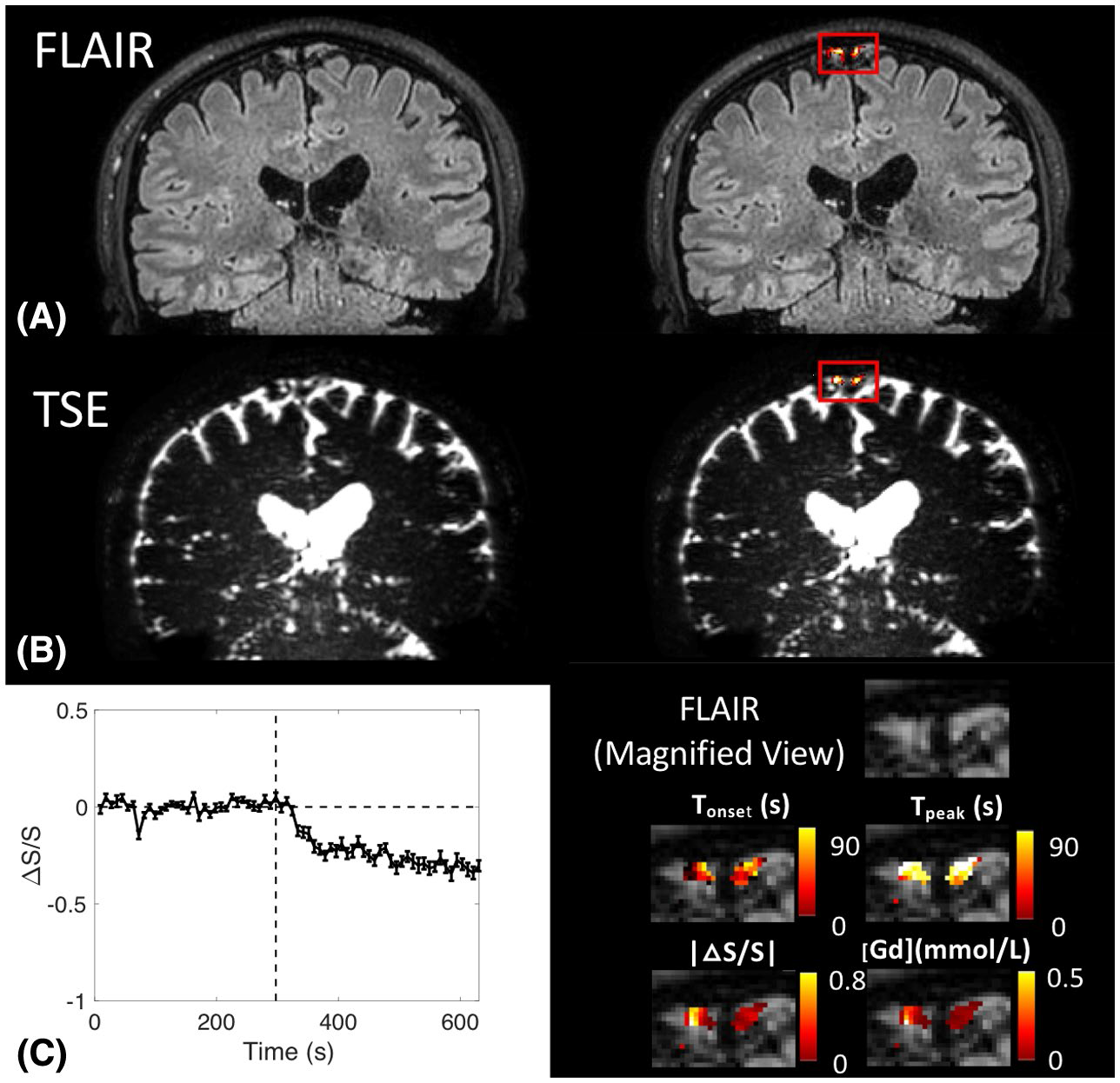
Figure from Cao et al., Magn Reson Med. 2020 Jul 3;. doi: 10.1002/mrm.28389.
Selected Publications:
- Cao D, Kang N, Pillai JJ, Miao X, Paez A, Xu X, Xu J, Li X, Qin Q, Van Zijl PCM, Barker P, Hua J*. Fast whole brain MR imaging of dynamic susceptibility contrast changes in the cerebrospinal fluid (cDSC MRI). Magn Reson Med. 2020 Jul 3;. doi: 10.1002/mrm.28389. [Epub ahead of print] PubMed PMID: 32621291.
5. Ultra-High Magnetic Field (7T) MRI and laminar fMRI
Ultra-high magnetic field (7T and above) is expect to significantly boost the intrinsic sensitivity of MRI, but also poses significant technical challenges. Since the arrival of the 7T human MRI scanner at Johns Hopkins in 2009, we have been working extensively on the development of functional and physiological MRI techniques on 7T. In addition to routine high resolution structural MRI scans such as MP2RAGE and FLAIR, we devised, improved and optimized several advanced MRI pulse sequences for 7T applications, including VASO, CEST and BOLD MRI. These methods have now been used in a number of studies on 7T MRI scanners at Johns Hopkins, as well as several other institutes in the US and abroad.
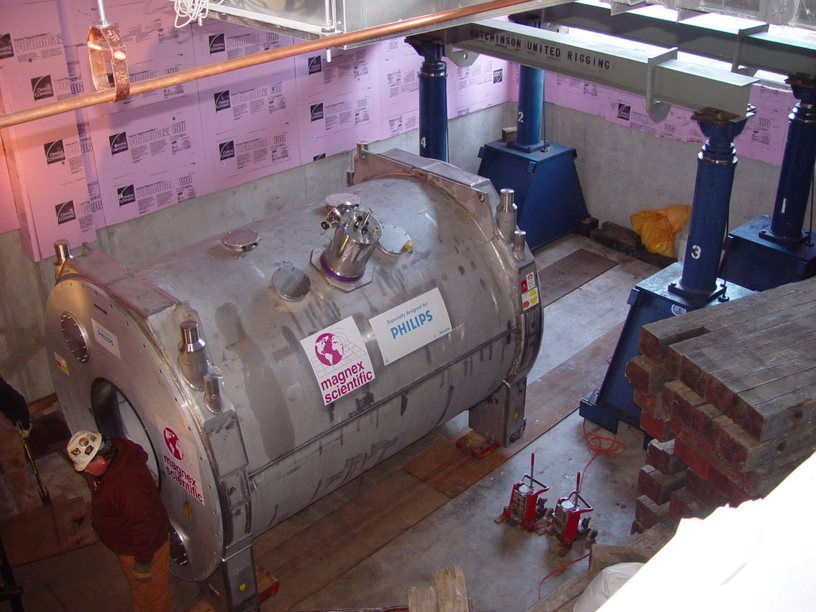
Figure: Delivery of our 7T magnet at the F.M. Kirby Research Center for Functional Brian Imaging on Sunday Nov 23 2009.
Laminar fMRI at 7T
The enhanced sensitivity at 7T enables the performance of whole brain human fMRI at a sub-millimeter (sub-mm) spatial resolution. In the human brain, the thickness of functionally distinct layers is usually sub-mm. In hierarchically organized brain systems, input and output signals arrive in different layers of a given functional unit. The distance between such input and output layers is in the range of 1-2mm in most brain regions. Therefore, sub-mm fMRI allows the detection of neuronal activities at the mesoscopic spatial regime of cortical layers, namely laminar (or layer-dependent or cortical-depth-dependent) fMRI. Such layer specific information can be used to infer directionality in brain circuits, whereas conventional fMRI methods performed at the macroscopic scale can only provide correlational information.
The ability to resolve layer specific activities in brain circuits is of utmost importance for neuroscience studies in healthy brains, as well as clinical studies attempting to understand the mechanisms of brain diseases. For instance, the memory circuit is well-known to be affected in many brain diseases, such as dementia. Several models have been propose to understand the information flow between different sub-regions in the medial temporal lobe (MTL), a structure that plays a central role in the memory circuit. Laminar fMRI offers the potential to probe the directionality of the information flow through the detection of neuronal activities in different layers of the entorhinal cortex (ERC, a key sub-region in the MTL) predominantly responsible for encoding and retrieval. To distinguish these segregated functions in the memory circuit is essential for studying the mechanisms and for understanding potential disease specific changes in the memory system. Using T2prep BOLD fMRI performed at sub-mm spatial resolution at 7T during a memory task, differential laminar activities were detected in the superficial and deep layers of the ERC associated with information encoding and retrieval in healthy human brains.
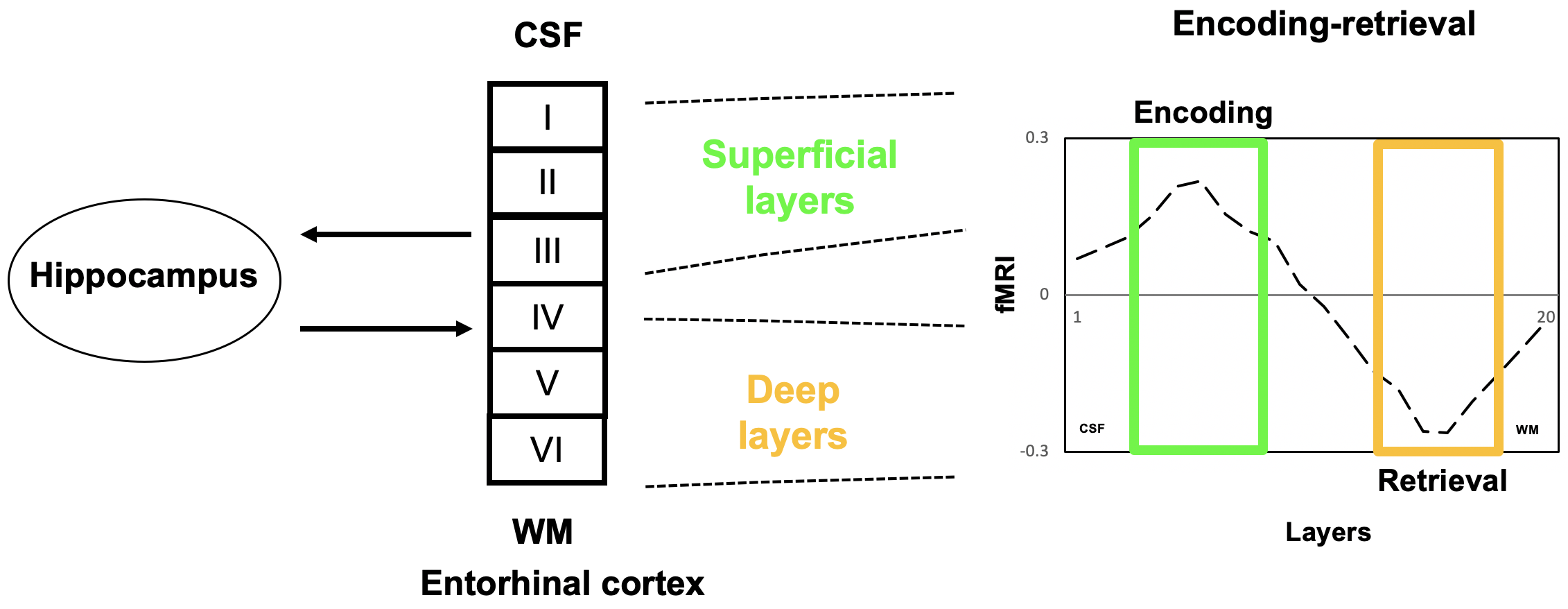
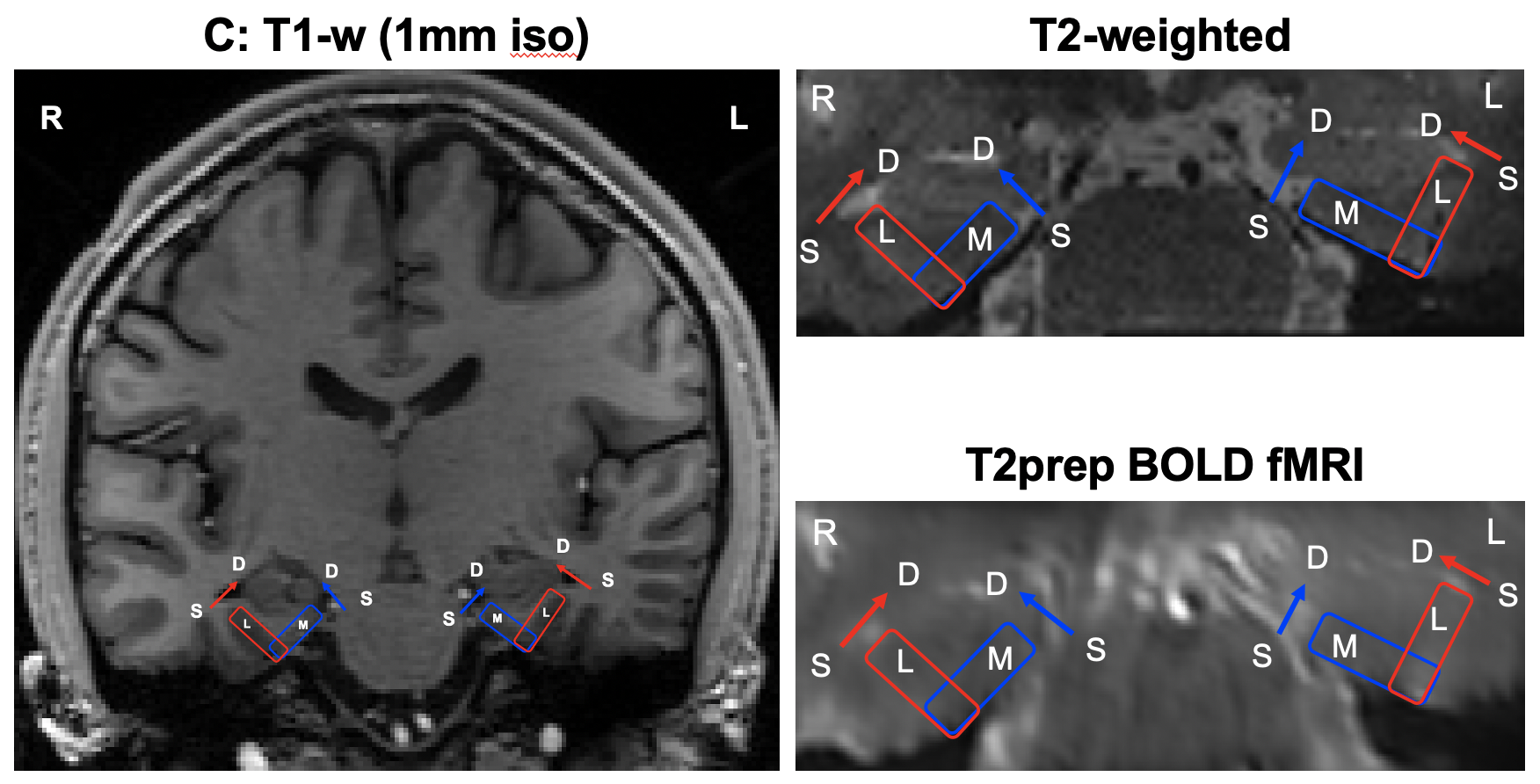
Figure: Anatomical location of the medial and lateral ERC. Coronal views of T1-weighted (1mm iso-tropic voxel, whole brain), T2-weighted (0.5mm isotropic voxel, partial brain) and T2prep BOLD fMRI images (0.9mm isotropic voxel, partial brain) are shown. R: right, L: left; S: superficial layer, D: deep layer; M: medial, L: lateral. The regions close to the CSF and WM are the superficial and deep layers, respectively.
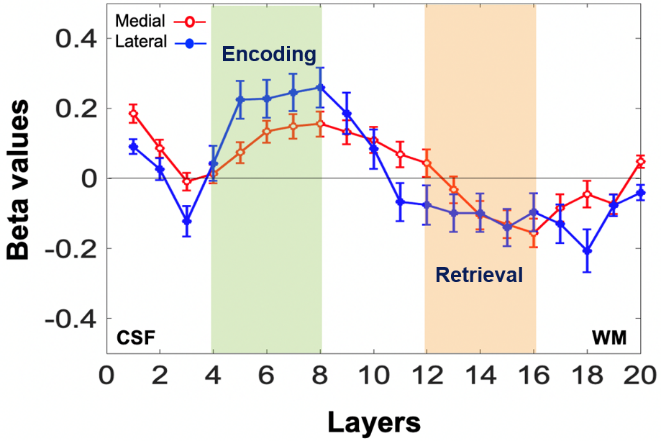
Figure: Average laminar activation (beta) profile in the lateral and medial ERC (n=9). The y-axis is the beta value for the contrast between successful encoding and retrieval trials. The x-axis is the layer numbers. Layers close to CSF and WM are superficial and deep layers, respectively. The beta values in the shaded layers were averaged to give mean beta values in the superficial (green) and deep (orange) layers, respectively. The error bars indicate the inter-subject standard errors.
Selected Publications:
- Hua J, Jones CK, Qin Q, Van Zijl PCM. Implementation of Vascular-space-occupancy (VASO) MRI at 7 Tesla. Magnetic Resonance in Medicine 69(4):1003-13, 2013. PMC4121129.
- Hua J, Qin Q, Van Zijl PCM, Pekar JJ, Jones CK. Whole-brain three-dimensional T2-weighted BOLD functional magnetic resonance imaging at 7 Tesla. Magnetic Resonance in Medicine 2014 Dec;72(6):1530-40. PMC4055555.
- Cheng Y, Van Zijl PCM, Hua J*. Measurement of Parenchymal Extravascular R2* and Tissue Oxygen Extraction Fraction Using Multi-echo VASO MRI at 7 Tesla. NMR in Biomedicine 2015 Feb;28(2):264-71. PMC4297270.
- Wu Y, Agarwal S, Jones CK, Webb AG, Van Zijl PCM, Hua J*, Pillai J. Measurement of Arteriolar Blood Volume in Brain Tumors Using MRI Without Exogenous Contrast Agent Administration at 7T. Journal of Magnetic Resonance Imaging 2016 Nov;44(5):1244-1255. PMC5045323.
- Hua J, Brandt AS, Lee S, Blair NIS, Wu Y, Su L, Patel J, Faria AV, Lim IAL, Unschuld PG, Pekar JJ, Van Zijl PCM, Ross CA, Margolis RL. Abnormal Grey Matter Arteriolar Cerebral Blood Volume in Schizophrenia Measured With 3D Inflow-Based Vascular-Space-Occupancy MRI at 7T. Schizophrenia Bulletin, 2017 May 1;43(3):620-632. doi:10.1093/schbul/sbw109. PMC5464028.
- Hua J, Blair NIS, Paez A, Choe A, Barber AD, Brandt A, Lim IAL, Xu F, Kamath V, Pekar JJ, van Zijl PCM, Ross CA, Margolis RL. Altered functional connectivity between sub-regions in the thalamus and cortex in schizophrenia patients measured by resting state BOLD fMRI at 7T. Schizophr Res. 2019 Apr;206:370-377. doi: 10.1016/j.schres.2018.10.016. Epub 2018 Nov 6. PubMed PMID: 30409697; PubMed Central PMCID: PMC6500777
Applications in Brain diseases: Selected Publications
Huntington’s Disease (HD)
- Unschuld PG, Liu X, Shanahan M, Margolis RL, Bassett SS, Brandt J, Schretlen DJ, Redgrave GW, Hua J, Hock C, Reading SA, Van Zijl PCM, Pekar JJ, Ross CA. Altered prefrontal network connectivity during a motor planning task in prodromal Huntington's disease. Cortex 49(10):2661-73, 2013. PMID23906595.
- Hua J, Unschuld PG, Margolis RL, Van Zijl PCM, Ross CA. Elevated Arteriolar Cerebral Blood Volume in Prodromal Huntington’s Disease Patients. Movement Disorders 29(3):396-401 2014. PMC3834086.
- Van Bergen JMG, Hua J, Unschuld PG, Lim IAL, Jones CK, Margolis RL, Ross CA, Van Zijl PCM, Li X. Quantitative susceptibility mapping suggests altered brain iron in premanifest Huntington’s disease. American Journal of Neuroradiology 2016 May;37(5):789-96. PMC4867278.
- Chen L, Hua J, Ross CA, Cai S, van Zijl PCM, Li X. Altered brain iron content and deposition rate in Huntington's disease as indicated by quantitative susceptibility MRI. J Neurosci Res. 2019 Apr;97(4):467-479. doi: 10.1002/jnr.24358. Epub 2018 Nov 29. PMID: 30489648. PMC6367012.
- Kronenbuerger M, Hua J*, Bang JYA, Ultz KE, Miao X, Zhang X, Pekar JJ, Van Zijl PCM, Duan W, Margolis RL, Ross CA. Differential Changes in Functional Connectivity of Striatum-Prefrontal and Striatum-Motor Circuits in Premanifest Huntington's Disease. Neurodegener Dis. 2019 Aug 14:1-10. doi: 10.1159/000501616. PMID: 31412344.
- Klinkmueller P, Kronenbuerger M, Miao X, Bang J, Ultz KE, Paez A, Zhang X, Duan W, Margolis RL, Zijl PCV, Ross CA, Hua J*. Impaired response of cerebral oxygen metabolism to visual stimulation in Huntington's disease. J Cereb Blood Flow Metab. 2020 Aug 17;:271678X20949286. doi: 10.1177/0271678X20949286. [Epub ahead of print] PubMed PMID: 32807001.
Alzheimer’s Disease (AD)
- van Bergen JM, Li X, Hua J, Schreiner SJ, Steininger SC, Quevenco FC, Wyss M, Gietl AF, Treyer V, Leh SE, Buck F, Nitsch RM, Pruessmann KP, van Zijl PC, Hock C, Unschuld PG. Colocalization of cerebral iron with Amyloid beta in Mild Cognitive Impairment. Sci Rep. 2016 Oct 17;6:35514. PMC5066274.
- Quevenco FC, Preti MG, van Bergen JM, Hua J, Wyss M, Li X, Schreiner SJ, Steininger SC, Meyer R, Meier IB, Brickman AM, Leh SE, Gietl AF, Buck A, Nitsch RM, Pruessmann KP, van Zijl PC, Hock C, Van De Ville D, Unschuld PG. Memory performance-related dynamic brain connectivity indicates pathological burden and genetic risk for Alzheimer's disease. Alzheimers Res Ther. 2017 Mar 31;9(1):24. PMC5374623.
- Hua J, Lee S, Blair NIS, Wyss M, van Bergen JMG, Schreiner SJ, Kagerer SM, Leh SE, Gietl AF, Treyer V, Buck A, Nitsch RM, Pruessmann KP, Lu H, Van Zijl PCM, Albert M, Hock C, Unschuld PG. Increased cerebral blood volume in small arterial vessels is a correlate of amyloid-β-related cognitive decline. Neurobiol Aging 76:181-193, 2019 Jan. PMID: 30738323. PMC6438210.
Schizophrenia
- Hua J, Brandt AS, Lee S, Blair NIS, Wu Y, Su L, Patel J, Faria AV, Lim IAL, Unschuld PG, Pekar JJ, Van Zijl PCM, Ross CA, Margolis RL. Abnormal Grey Matter Arteriolar Cerebral Blood Volume in Schizophrenia Measured With 3D Inflow-Based Vascular-Space-Occupancy MRI at 7T. Schizophrenia Bulletin, 2017 May 1;43(3):620-632. doi:10.1093/schbul/sbw109. PMC5464028.
- Brandt AS, Unschuld PG, Lim IAL, Churchill G, Harris A, Pradhan S, Hua J, Barker P, Ross CA, Van Zijl PCM, Edden R, Margolis RL. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: A 1H MRS Study at 7 Tesla. Schizophrenia Research 2016 Apr;172(1-3):101-5. PMC4821673.
- Hua J, Blair NIS, Paez A, Choe A, Barber AD, Brandt A, Lim IAL, Xu F, Kamath V, Pekar JJ, van Zijl PCM, Ross CA, Margolis RL. Altered functional connectivity between sub-regions in the thalamus and cortex in schizophrenia patients measured by resting state BOLD fMRI at 7T. Schizophr Res. 2019 Apr;206:370-377. doi: 10.1016/j.schres.2018.10.016. Epub 2018 Nov 6. PubMed PMID: 30409697; PubMed Central PMCID: PMC6500777
Presurgical Brain Mapping in Brain Tumor and Epilepsy
- Zaca D, Hua J, Pillai JJ. Cerebrovascular reactivity mapping for brain tumor presurgical planning. World J Clin Oncol. 2:289-98, 2011. PMC3139032.
- Wu Y, Agarwal S, Jones CK, Webb AG, Van Zijl PCM, Hua J*, Pillai J. Measurement of Arteriolar Blood Volume in Brain Tumors Using MRI Without Exogenous Contrast Agent Administration at 7T. Journal of Magnetic Resonance Imaging 2016 Nov;44(5):1244-1255. PMC5045323.
- Agarwal S, Sair HI, Airan R, Hua J, Jones CK, Heo HY, Olivi A, Lindquist MA, Pekar JJ, Pillai J. Demonstration of brain tumor-induced neurovascular uncoupling in resting state fMRI at ultra-high field. Brain Connectivity 2016 May;6(4):267-72. PMID26918887.
- Hua J, Miao X, Agarwal S, Bettegowda C, Quiñones-Hinojosa A, Laterra J, Van Zijl PCM, Pekar JJ, Pillai JJ. Language mapping using T2-prepared BOLD functional MRI in the presence of large susceptibility artifacts – initial results in brain tumor and epilepsy patients. Tomography 2017 Jun;3(2):105-113. DOI: 10.18383/j.tom.2017.00006. PMC5552052.
- Agarwal S, Hua J, Sair HI, Gujar S, Bettegowda C, Lu H, Pillai JJ. Repeatability of language fMRI lateralization and localization metrics in brain tumor patients. Human Brain Mapping 2018 Dec;39(12):4733-4742. doi: 10.1002/hbm.24318. PMID: 30076768. PMC6218318.
- Agarwal S, Sair HI, Gujar S, Hua J, Lu H, Pillai JJ. Functional Magnetic Resonance Imaging Activation Optimization in the Setting of Brain Tumor-Induced Neurovascular Uncoupling Using Resting-State Blood Oxygen Level-Dependent Amplitude of Low Frequency Fluctuations. Brain Connect. 2019 Feb 28. doi: 10.1089/brain.2017.0562. PMID: 30547681.
Current Appointment:
Kennedy Krieger Institute
F.M. Kirby Research Center for Functional Brain Imaging
The Johns Hopkins University School of Medicine, Baltimore, MD
Associate Professor
Department of Radiology and Radiological Sciences
Contact Information
Office Address:
The F. M. Kirby Research Center for Functional Brain Imaging
707 N. Broadway, Room G-25
Baltimore, MD 21205
Contact: Heather Mackey, 443-923-9500
Email: jhua1@jhmi.edu
Office Phone: 443-923-3848
Publications
For a list of Dr. Hua's most recent publications searched from the NIH's PubMed Database, please use this Search Link.
